Peginterferon alfa-2a
About Medicine
Pegaferon® (Peginterferon alfa-2a) is a recombinant human protein (MW 19 kDa) conjugated to monomethoxy polyethylene glycol (PEG) for a total MW of about 60 kDa. Interferon alfa-2a is produced biosynthetically using recombinant DNA technology and is the product of a cloned human leukocyte interferon gene inserted into and expressed in E coli. Purified IFN has 165 amino acids and a molecular mass of 19237.
Prefilled syringes: 180 μg Peginterferon alfa-2a in 0.5 mL
Sterile solution, clear and colorless in prefilled syringe.
Overdosage
If you think that you have used too much or too little Pegaferon®, tell your doctor, nurse or pharmacist immediately, even if you have no signs of a problem.
What if you forget to use Pegaferon®?
If you realize you missed your injection 1 or 2 days after it was scheduled, you should inject your recommended dose as soon as possible. Take your next injection on the regularly scheduled day.
If you realize you missed your injection 3 to 5 days after it was scheduled, you should take your injection at the recommended dose as soon as possible. Take your next doses at 5 day intervals until you return to your regularly scheduled day of the week.
As an example: Your regular weekly Pegaferon® injection is on Monday. You remember on Friday that you forgot to take your injection on Monday (4 days late). You should inject your regularly scheduled dose immediately on Friday and take your next injection on Wednesday (5 days after your Friday dose). Your next injection will be on the Monday, 5 days later after the Wednesday injection. You are now back on your regularly scheduled day and should continue your injections every Monday.
If you realize you missed your injection 6 days after it was scheduled, you should wait and take your dose on the next day, your regularly scheduled day.
Contact your doctor or pharmacist if you need any help determining how to manage a missed dose of Pegaferon®.
Do not take a double dose to make up for a forgotten dose.
Effect on Ability to Drive and Operate Machinery
Pegaferon® has minor or moderate influence on the ability to drive and use machines. Patients who develop dizziness, confusion, somnolence or fatigue should be cautioned to avoid driving or operating machinery.
Chronic Hepatitis C (CHC)
Pegaferon® (peginterferon alfa-2a) is indicated for the treatment of Chronic Hepatitis C in:
Adult patients without cirrhosis.
Adult patients with compensated cirrhosis, including HCV/HIV co-infection patients with stable HIV disease with or without antiretroviral therapy.
Chronic Hepatitis B (CHB)
Pegaferon® is indicated for the treatment of both HBeAg-positive and HBeAg-negative chronic hepatitis B in patients with compensated liver disease, liver inflammation and evidence of viral replication (both cirrhotic and non-cirrhotic disease).
Geriatrics (> 65 years of age)
Clinical studies of Pegaferon® alone or in combination with ribavirin did not include sufficient numbers of subjects aged 65 or over to determine whether they respond differently from younger subjects.
Pediatrics (< 18 years of age)
Pegaferon® is not authorized for use in children and adolescents under the age of 18 years.
Chronic hepatitis B – adult patients
The recommended dosage and duration of Pegaferon® for both HBeAg-positive and HBeAg-negative chronic hepatitis B is 180 micrograms once weekly for 48 weeks by subcutaneous administration in the abdomen or thigh.Chronic hepatitis C – treatment-naïve adult patients
The recommended dose for Pegaferon® is 180 micrograms once weekly by subcutaneous administration in the abdomen or thigh given in combination with oral ribavirin or as monotherapy.Duration of treatment–dual therapy with Pegaferon® and ribavirin
The duration of combination therapy with ribavirin for chronic hepatitis C depends on viral genotype. Patients infected with HCV genotype 1 who have detectable HCV RNA at week 4 regardless of pre-treatment viral load should receive 48 weeks of therapy.
Treatment for 24 weeks may be considered in patients infected with:
– genotype 1 with low viral load (LVL) ( ≤ 800,000 IU/mL) at baseline or
– genotype 4 who become HCV RNA negative at week 4 and remain HCV RNA negative at week 24. However, overall 24 weeks treatment duration may be associated with a higher risk of relapse than 48 weeks treatment duration. In these patients, tolerability to combination therapy and additional prognostic factors such as degree of fibrosis should be taken into account when deciding on treatment duration.Shortening the treatment duration in patients with genotype 1 and high viral load (HVL) (>800, 000 IU/mL) at baseline who become HCV RNA negative at week 4 and remain HCV RNA negative at week 24 should be considered with even more caution since the limited data available suggest that this may significantly negatively impact the sustained virologic response.
Patients infected with HCV genotype 2 or 3 who have detectable HCV RNA at week 4, regardless of pre-treatment viral load should receive 24 weeks of therapy.
Treatment for only 16 weeks may be considered in selected patients infected with genotype 2 or 3 with LVL (≤ 800,000 IU/mL) at baseline who become HCV negative by week 4 of treatment and remains HCV negative by week 16. Overall 16 weeks of treatment may be associated with a lower chance of response and is associated with a higher risk of relapse than 24 week treatment duration. In these patients, tolerability to combination therapy and the presence of additional clinical or prognostic factors such as degree of fibrosis should be taken into account when considering deviations from standard 24 weeks treatment duration.
Shortening the treatment duration in patients infected with genotype 2 or 3 with HVL (> 800,000 IU/mL) at baseline who become HCV negative by week 4 should be considered with more caution as this may significantly negatively impact the sustained virological response.
Available data for patients infected with genotype 5 or 6 are limited; therefore combination treatment with 1,000/1,200 mg of ribavirin for 48 weeks is recommended.
The recommended duration of Pegaferon® monotherapy is 48 weeks.
Chronic hepatitis C – treatment-experienced adult patients
The recommended dose of Pegaferon® in combination with ribavirin is 180 mcg once weekly by subcutaneous administration. For patients <75 kg and ≥75 kg, 1000 mg daily and 1200 mg daily of ribavirin,respectively, and regardless of genotype, should be administered. Patients who have detectable virus at week 12 should stop therapy. The recommended total duration of therapy is 48 weeks. If patients infected with virus genotype 1, not responding to prior treatment with peginterferon and ribavirin are considered for treatment, the recommended total duration of therapy is 72 weeks.
HIV-HCV co-infected adult patients
The recommended dosage for Pegaferon®, alone or in combination with ribavirin is 180 micrograms once weekly subcutaneously for 48 weeks. For patients infected with HCV genotype 1 <75 kg and ≥75kg, 1000 mg daily and 1200 mg daily of ribavirin,respectively, should be administered. Patients infected with HCV genotypes other than genotype 1 should receive 800 mg daily of ribavirin. Duration of therapy less than 48 weeks has not been adequately studied.
Predictability of response and non-response with Pegaferon® and ribavirindual therapy – treatment-naïve patients
Early virological response by week 12, defined as a 2 log viral load decrease or undetectable levels of HCV RNA has been shown to be predictive for sustained response.
Table 1: Predictive value of week 12 virological response at the recommended dosing regimen while on Pegaferon® combination therapy:
Positive Negative Genotype Predictive Value Sustained response Response by week 12 Predictive Value No sustained response No response by week 12 58%(271/467) 271 467 95%(97/102) 97 102 Genotype 1(N=569) 87%(81/93) 81 93 100%(3/3) 3 3 Genotype 2 and 3(N=96) The negative predictive value for sustained response in patients treated with Pegaferon® in monotherapy was 98%.
Predictability of response and nonresponse with Pegaferon® and ribavirin dual therapy – treatment experienced patients
In nonresponder patients retreated for 48 or 72 weeks, viral suppression at week 12 (undetectable HCV RNA defined as <50 IU/mL) has been shown to be predictive for sustained virological response. The probabilities of not achieving a sustained virological response with 48 or 72 weeks of treatment if viral suppression was not achieved at week 12 were 96% (363 of 380) and 96% (324 of 339), respectively. The probabilities of achieving a sustained virological response with 48 or 72 weeks of treatment if viral suppression was achieved at week 12 were 35% (20 of 57) and 57% (57 of 100), respectively.
General
Where dose adjustment is required for moderate to severe adverse reactions (clinical and/or laboratory) initial dose reduction to 135 micrograms is generally adequate for adult patients. In some cases, dose reduction to 90 micrograms or 45 micrograms is necessary. Dose increases to or towards the original dose may be considered when the adverse reaction abates.
Hematological
For adults, dose reduction is recommended if the neutrophil count is < 750/mm3. For patients with Absolute Neutrophil Count (ANC) < 500/mm3 treatment should be suspended until ANC values return to > 1000/mm3. Therapy should initially be reinstituted at 90 micrograms Pegaferon® and the neutrophil count monitored. Dose reduction to 90 micrograms is recommended if the platelet count is < 50,000/mm3. Cessation of therapy is recommended when platelet count decreases to levels < 25,000/mm3. Specific recommendations for management of treatment-emergent anemia in adults are as follows: ribavirin should be reduced to 600 milligrams/day (200 milligrams in the morning and 400 milligrams in the evening) if either of the following apply: (1) a patient without significant cardiovascular disease experiences a fall in hemoglobin to < 10 g/dl and ≥ 8.5 g/dl, or (2) a patient with stable cardiovascular disease experiences a fall in hemoglobin by ≥ 2 g/dl during any 4 weeks of treatment. A return to original dosing is not recommended. Ribavirin should be discontinued if either of the following applies: (1) a patient without significant cardiovascular disease experiences a fall in hemoglobin confirmed to < 8.5 g/dl; (2) A patient with stable cardiovascular disease maintains a hemoglobin value < 12 g/dl despite 4 weeks on a reduced dose. If the abnormality is reversed, ribavirin may be restarted at 600 milligrams daily, and further increased to 800 milligrams daily at the discretion of the treating physician. A return to original dosing is not recommended.
Table 2: Dose adjustment for hematologic adverse reaction:
Discontunue combination Withhold Peginterferon alfa 2-a Reduce Peginterferon alfa 2-a to 135/90/45 micrograms Withhold ribavirin Reduce ribavirin to 600 mg <500/mm3 <750/ mm3 Absolute Neutrophil Count < 25,000/mm3 < 50,000/mm3> 25,000/mm3 Platelet Count < 8.5 g/dL < 10 g/dL, and ≥ 8.5 g/dL HemoglobinNo cardiac disease < 12 g/dL despite 4 weeks at reduced dose Decrease ≥2g/dL during any 4 weeks HemoglobinStable cardiac disease Liver function
Fluctuations in abnormalities of liver function tests are common in patients with chronic hepatitis C. Increases in ALT levels above baseline (BL) have been observed in patients treated with Pegaferon® including patients with a virological response. In chronic hepatitis C clinical trials with adult patients, isolated increases in ALT (≥ 10x ULN, or ≥ 2x BL for patients with a BL ALT ≥ 10x ULN) which resolved without dose modification were observed in 8 of 451 patients treated with combination therapy. If ALT increase is progressive or persistent, the dose should be reduced initially to 135 micrograms. When increases in ALT levels are progressive despite dose reduction, or are accompanied by increased bilirubin or evidence of hepatic decompensation, therapy should be discontinued.
For chronic hepatitis B patients, transient flares of ALT levels sometimes exceeding 10 times the upper limit of normal are not uncommon, and may reflect immune clearance. Treatment should normally not be initiated if ALT is >10 times the upper limit of normal. Consideration should be given to continuing treatment with more frequent monitoring of liver function during ALT flares. If the Pegaferon® dose is reduced or withheld, therapy can be restored once the flare is subsiding.Special populations
Older people
Adjustments in the recommended dosage of 180 micrograms once weekly are not necessary when instituting Pegaferon® therapy in elderly patients.
Renal impairment
In patients with end stage renal disease, a starting dose of 135 micrograms should be used. Regardless of the starting dose or degree of renal impairment, patients should be monitored and appropriate dose reductions of Pegaferon® during the course of therapy should be made in the event of adverse reactions.
Hepatic impairment
In patients with compensated cirrhosis (e.g., ChildPugh A), Pegaferon® has been shown to be effective and safe. Pegaferon® has not been evaluated in patients with decompensated cirrhosis (e.g., ChildPugh B or C or bleeding esophageal varices).
Pegaferon® is contraindicated in neonates and young children up to 3 years old due to the excipient benzyl alcohol. For children and adolescents aged 5 to 17 years with chronic hepatitis C, and having a Body Surface Area (BSA) greater than 0.7 m2.
Patients who initiate treatment prior to their 18th birthday should maintain pediatric dosing through the completion of therapy.
Duration of treatment for pediatric
The duration of treatment with Pegaferon® in combination with ribavirin in pediatric patients with chronic hepatitis C depends on viral genotype. Patients infected with viral genotypes 2 or 3 should receive 24 weeks of treatment, while patients infected with any other genotype should receive 48 weeks of therapy. Patients, who still have detectable levels of HCV RNA despite an initial 24 weeks of therapy, should discontinue therapy, as it is unlikely they will be able to achieve a sustained virological response with continued therapy.
Table 3: Pegaferon® dosing recommendations for pediatric patients aged 5 to 17 years
Weekly dose (mcg) Body Surface Area (BSA) range (m2) 65 0.71-0.74 90 0.75-1.08 135 1.09-1.51 180 >1.51 Dose adjustment for adverse reactions in pediatric patients
For pediatric patients, based on toxicities (see Table 4), up to three levels of dose modification can be made before dose interruption or discontinuation is considered.
Table 4: Dose modification recommendations in pediatric patients
3 level reduction (mcg) 2 level reduction (mcg) 1 level reduction (mcg) Starting dose (mcg) 20 30 45 65 20 45 65 90 30 65 90 135 45 90 135 180 If toxicities occur which may be related to Pegaferon® and/or ribavirin administration, the dose of one or both medicinal products can be reduced. Additionally, ribavirin or Pegaferon® plus ribavirin combination therapy can be discontinued. It is important to note that ribavirin should never be given as monotherapy. Recommendations for dose modifications for toxicities known to have an association with Pegaferon® administration that are specific for the pediatric population are presented in Table 5. Unless otherwise noted, the management of all other toxicities should follow the adult recommendations.
Table 5: Dose modification recommendations for toxicities in pediatric patients
Peginterferon alfa 2-a dose modification Toxicity - 750-999 cells/mm3: Week 1-2 – immediate 1 level adjustment; Week 3-48: no modification.
- 500-749 cells/ mm3: Week 1-2 – interrupt dosing until>750 cells/ mm3 then resume dose with a 1 level adjustment, assess weekly for the next 3 weeks to verify ANC>750 cells/ mm3; Week 3-48 – immediate 1 level adjustment.
- 250-499 cells/ mm3: Week 1-2 – interrupt dosing until>750 cells/ mm3 then resume dose with a 2 level adjustment, Week 3 -48 – interrupt dosing until >750 cells/mm3 then resume dose with a 1 level adjustment.
- < 250 cells/mm3 (or febrile neutropenia) discontinue treatment.
Neutropenia - For persistent or increasing elevations ≥5 but <10× ULN, reduce dose with a 1 level adjustment and monitor weekly ALT level to ensure it is stable or decreasing.
- For persistent ALT values ≥10× ULN discontinue treatment.
Increase alanine transaminase (ALT) There is limited experience with Pegaferon® in treating pediatric patients with HCV aged 3 to 5 years, or who have failed to be adequately treated previously. There are no data in pediatric patients coinfected with HCV/HIV or with renal impairment.
Method of administration
Pegaferon® is administered subcutaneously in the abdomen or thigh. Pegaferon® is designed for administration by the patient or carer. Each syringe should be used by one person only and is for single use. Appropriate training is recommended for nonhealthcare professionals administering this medicinal product.
Hypersensitivity to the active substance, to Alfa interferons, or to any of the excipients.
Autoimmune hepatitis:
– Severe hepatic dysfunction or decompensated cirrhosis of the liver.
– A history of severe preexisting cardiac disease, including unstable or uncontrolled cardiac disease in the previous six months.HIV HCV patients with cirrhosis and a ChildPugh score ≥ 6, except if only due to indirect hyperbilirubinemia caused by medicinal products such as atazanavir and indinavir.
Combination with telbivudine.
Neonates and young children up to 3 years old, because of the excipient benzyl alcohol.
– In pediatric patients, the presence of, or history of severe psychiatric condition, particularly severe depression, suicidal ideation or suicidal attempt.
Psychiatric and Central Nervous System (CNS): Severe CNS effects, particularly depression, suicidal ideation and attempted suicide have been observed in some patients during peginterferon therapy and even after treatment discontinuation mainly during the 6month follow up period. Other CNS effects including aggressive behavior (sometimes directed against others such as homicidal ideation), bipolar disorders, mania, confusion and alterations of mental status have been observed with alfa interferons. All patients should be closely monitored for any signs or symptoms of psychiatric disorders. If symptoms of psychiatric disorders appear, the potential seriousness of these undesirable effects must be borne in mind by the prescribing physician and the need for adequate therapeutic management should be considered. If psychiatric symptoms persist or worsen, or suicidal ideation is identified, it is recommended that treatment with Pegaferon® be discontinued, and the patient followed, with psychiatric intervention as appropriate.
Patients with existence of, or history of severe psychiatric conditions:If treatment with Pegaferon® is judged necessary in patients with existence or history of severe psychiatric conditions, this should only be initiated after having ensured appropriate individualized diagnostic and therapeutic management of the psychiatric condition.
The use of Pegaferon® in children and adolescents with existence of or history of severe psychiatric conditions is contraindicated.
Patients with substance use/abuse:HCV infected patients having a co-occurring substance use disorder (alcohol, cannabis, etc.) are at an increased risk of developing psychiatric disorders or exacerbation of already existing psychiatric disorders when treated with alfa interferon. If treatment with alfa interferon is judged necessary in these patients, the presence of psychiatric comorbidities and the potential for other substance use should be carefully assessed and adequately managed before initiating therapy. If necessary, an interdisciplinary approach including a mental health care provider or addiction specialist should be considered to evaluate, treat and follow the patient. Patients should be closely monitored during therapy and even after treatment discontinuation. Early intervention for reemergence or development of psychiatric disorders and substance use is recommended.
Growth and development (children and adolescents): During the course of Pegaferon® plus ribavirin therapy lasting up to 48 weeks in patients aged 5 to 17 years, weight loss and growth inhibition were common. The expected benefit of treatment should be carefully weighed against the safety findings observed for children and adolescents in the clinical trials on a case by case basis. It is important to consider that the combination therapy induced a growth inhibition during treatment, the reversibility of which is uncertain. This risk should be weighed against the disease characteristics of the child, such as evidence of disease progression (notably fibrosis), comorbidities that may negatively influence the disease progression (such as HIV coinfection), as well as prognostic factors of response (HCV genotype and viral load). Whenever possible the child should be treated after the pubertal growth spurt, in order to reduce the risk of growth inhibition. There are no data on long term effects on sexual maturation.
In order to improve the traceability of biological medicinal products, the trade name of the administered product should be clearly recorded (or stated) in the patient file.
In clinical trials, Pegaferon® treatment was associated with decreases in both total white blood cell (WBC) count and absolute neutrophil count (ANC), usually starting within the first 2 weeks of treatment. Progressive decreases after 8 weeks of therapy were infrequent. The decrease in ANC was reversible upon dose reduction or cessation of therapy, reached normal values by 8 weeks in the majority of patients and returned to baseline in all patients after about 16 weeks.
Pegaferon® treatment has been associated with decreases in platelet count, which returned to pretreatment levels during the post-treatment observation period. In some cases, dose modification may be necessary.
The occurrence of anemia (hemoglobin <10 g/dl) has been observed in up to 15% of chronic hepatitis C patients in clinical trials on the combined treatment of Pegaferon® with ribavirin. The frequency depends on the treatment duration and the dose of ribavirin. The risk of developing anemia is higher in the female population.
Caution should be exercised when administering Pegaferon® in combination with other potentially myelosuppressive agents. Pancytopenia and bone marrow suppression have been reported in the literature to occur within 3 to 7 weeks after the administration of a peginterferon and ribavirin concomitantly with azathioprine. This myelotoxicity was reversible within 4 to 6 weeks upon withdrawal of HCV antiviral therapy and concomitant azathioprine and did not recur upon reintroduction of either treatment alone.
The use of Pegaferon® and ribavirin combination therapy in chronic hepatitis C patients who failed prior treatment has not been adequately studied in patients who discontinued prior therapy for hematological adverse reactions. Physicians considering treatment in these patients should carefully weigh the risks versus the benefits of retreatment.
Thyroid function abnormalities or worsening of preexisting thyroid disorders have been reported with the use of alfa interferons, including peginterferon. Prior to initiation of Pegaferon® therapy, TSH and T4 levels should be evaluated. Pegaferon® treatment may be initiated or continued if TSH levels can be maintained in the normal range by pharmaceutical means. TSH levels should be determined during the course of therapy if a patient develops clinical symptoms consistent with possible thyroid dysfunction.
Hypoglycemia, hyperglycemia and diabetes mellitus have been observed with peginterferon. Patients with these conditions who cannot be effectively controlled by medication should not begin Pegaferon® monotherapy or Pegaferon®/ribavirin combination therapy. Patients who develop these conditions during treatment and cannot be controlled with medication should discontinue Pegaferon® or Pegaferon®/ribavirin therapy.
Hypertension, supraventricular arrhythmias, congestive heart failure, chest pain and myocardial infarction have been associated with alfa interferon therapies, including Pegaferon®. It is recommended that patients who have preexisting cardiac abnormalities have an electrocardiogram prior to initiation of Pegaferon® If there is any deterioration of cardiovascular status, therapy should be suspended or discontinued. In patients with cardiovascular disease, anemia may necessitate dose reduction or discontinuation of ribavirin.
Retinopathies including retinal hemorrhages, cotton wool spots, papilloedema, optic neuropathy and retinal artery or vein obstruction which may result in loss of vision have been reported in rare instances with Pegaferon®. All patients should have a baseline eye examination. Any patient complaining of decrease or loss of vision must have a prompt and complete eye examination. Adult and pediatric patients with preexisting ophthalmologic disorders (e.g., diabetic or hypertensive retinopathy) should receive periodic ophthalmologic exams during Pegaferon® Pegaferon® treatment should be discontinued in patients who develop new or worsening ophthalmologic disorders.
Pulmonary symptoms, including dyspnea, pulmonary infiltrate, pneumonia, and pneumonitis has been reported during therapy with Pegaferon®. In case of persistent or unexplained pulmonary infiltrates or pulmonary function impairment, treatment should be discontinued.
Use of alfa interferons has been associated with exacerbation or provocation of psoriasis and sarcoidosis. Pegaferon® must be used with caution in patients with psoriasis, and in cases of onset or worsening of psoriatic lesions, discontinuation of therapy should be considered.
The safety and efficacy of Pegaferon® and ribavirin treatment have not been established in patients with liver and other transplantations. Liver and renal graft rejections have been reported with Pegaferon®, alone or in combination with ribavirin.
In patients who develop evidence of hepatic decompensation during treatment, Pegaferon® should be discontinued. Increases in ALT levels above baseline have been observed in patients treated with Pegaferon®, including patients with a viral response. When the increase in ALT levels is progressive and clinically significant, despite dose reduction, or is accompanied by increased direct bilirubin, therapy should be discontinued.
In chronic hepatitis B, unlike chronic hepatitis C, disease exacerbations during therapy are not uncommon and are characterized by transient and potentially significant increases in serum ALT. In clinical trials with Pegaferon® in HBV, marked transaminase flares have been accompanied by mild changes in other measures of hepatic function and without evidence of hepatic decompensation. In approximately half the cases of flares exceeding 10 times the upper limit of normal, Pegaferon® dosing was reduced or withheld until the transaminase elevations subsided, while in the rest therapy was continued unchanged. More frequent monitoring of hepatic function was recommended in all instances.
Serious, acute hypersensitivity reactions (e.g., urticaria, angioedema, bronchoconstriction, and anaphylaxis) have been rarely observed during alfa interferon therapy. If this occurs, therapy must be discontinued and appropriate medical therapy instituted immediately. Transient rashes do not necessitate interruption of treatment.
The development of autoantibodies and autoimmune disorders has been reported during treatment with alfa interferons. Patients predisposed to the development of autoimmune disorders may be at increased risk. Patients with signs or symptoms compatible with autoimmune disorders should be evaluated carefully, and the benefit-risk of continued interferon therapy should be reassessed.
Cases of Vogt-Koyanagi-Harada (VKH) syndrome have been reported in patients with chronic hepatitis C treated with interferon. This syndrome is a granulomatous inflammatory disorder affecting the eyes, auditory system, meninges, and skin. If VKH syndrome is suspected, antiviral treatment should be withdrawn and corticosteroid therapy discussed.
While fever may be associated with the flulike syndrome reported commonly during interferon therapy, other causes of persistent fever, particularly serious infections (bacterial, viral, fungal) must be ruled out, especially in patients with neutropenia. Serious infections (bacterial, viral, fungal) and sepsis have been reported during treatment with alfa interferons including peginterferon. Appropriate anti-infective therapy should be started immediately and discontinuation of therapy should be considered.
Patients coinfected with HIV and receiving Highly Active Anti-Retroviral Therapy (HAART) may be at increased risk of developing lactic acidosis. Caution should therefore be exercised when adding Pegaferon® and ribavirin to HAART therapy.
Coinfected patients with advanced cirrhosis receiving HAART may also be at increased risk of hepatic decompensation and possibly death if treated with ribavirin in combination with interferons, including Pegaferon®. Baseline variables in coinfected cirrhotic patients that may be associated with hepatic decompensation include: increased serum bilirubin, decreased hemoglobin, increased alkaline phosphatase or decreased platelet count, and treatment with didanosine (ddI).
During treatment, coinfected patients should be closely monitored for signs and symptoms of hepatic decompensation (including ascites, encephalopathy, variceal bleeding, impaired hepatic synthetic function; e.g., ChildPugh score of 7 or greater). The ChildPugh scoring may be affected by factors related to treatment (i.e. indirect hyperbilirubinemia, decreased albumin) and not necessarily attributable to hepatic decompensation. Treatment with Pegaferon® should be discontinued immediately in patients with hepatic decompensation.
In patients coinfected with HIV-HCV, limited efficacy and safety data are available in patients with CD4 counts less than 200 cells/μl. Caution is therefore warranted in the treatment of patients with low CD4 counts.
Dental and periodontal disorders, which may lead to loss of teeth, have been reported in patients receiving peginterferon and ribavirin combination therapy. In addition, dry mouth could have a damaging effect on teeth and mucous membranes of the mouth during long-term treatment with the combination of Pegaferon® and ribavirin.
Patients should brush their teeth thoroughly twice daily and have regular dental examinations. In addition some patients may experience vomiting. If this reaction occurs, they should be advised to rinse out their mouth thoroughly afterwards.
Use of peginterferon as long term maintenance monotherapy (unapproved use):
In a randomized, controlled US study (HALTC) of HCV nonresponder patients with varied degrees of fibrosis where 3.5 years of treatment with 90 micrograms/week of peginterferon monotherapy was studied, no significant reductions were observed in the rate of fibrosis progression or related clinical events.
There are no or limited amount of data from the use of peginterferon alfa2a in pregnant women. Studies in animals with interferon alfa-2a have shown reproductive toxicity (see section 5.3) and the potential risk for humans is unknown. Pegaferon® is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is unknown whether peginterferon alfa2a/metabolites are excreted in human milk. Because of the potential for adverse reactions in breastfed infants, breastfeeding should be discontinued prior to initiation of treatment.
- Very common (>10%):
- Anorexia
- Depression*
- Anxiety
- Insomnia*
- Headache
- Dizziness*
- Impaired concentration
- Dyspnea
- Cough
- Diarrhea*
- Nausea*
- Abdominal pain*
- Alopecia
- Dermatitis
- Pruritis
- Dry skin
- Myalgia
- Arthralgia
- Pyrexia,
- Rigors*
- Pain*
- Asthenia
- Fatigue
- Injection site reaction*
- Irritability*
*These adverse reactions were common (≥1/100 to <1/10) in CHB patients treated with peginterferon monotherapy.
Common (1-10%):
Bronchitis, Upper respiratory infection, Oral candidiasis, Herpes simplex, Fungal, Viral and bacterial infections, Thrombocytopenia, Anemia, Lymphadenopathy, Hypothyroidism, Hyperthyroidism, Aggression, Mood alteration, Emotional disorders, Nervousness, Libido decreased, Syncope, Migraine, Memory Impairment, Weakness, Hypoesthesia, Hyperesthesia, Paresthesia, Tremor, Taste disturbance, Nightmares, Somnolence, Vision blurred, Eye pain, Eye inflammation, Vertigo, Earache, Tachycardia, Edema peripheral, Palpitations, Flushing, Dyspnea, Exertional, Epistaxis, Nasopharyngitis, Sinus congestion, Nasal congestion, Rhinitis, Sore throat, Vomiting, Dyspepsia, Dysphagia, Mouth ulceration, Gingival bleeding, Glossitis, Stomatitis, Flatulence, Dry mouth, Psoriasis, Urticaria, Eczema, Rash, Sweating increased, Skin disorder, Photosensitivity reaction, Night sweats, Back pain, Arthritis, Muscle weakness, Bone pain, Neck pain, Musculoskeletal pain, Muscle cramps, Impotence, Chest pain, Influenza like illness, Malaise, Lethargy, Hot flushes, Thirst, Weight decreased
Uncommon (0.1-1%):
Pneumonia, Skin infection, Hepatic neoplasm, Sarcoidosis, Thyroiditis, Diabetes, Dehydration, Suicidal ideation, Hallucinations, Peripheral neuropathy, Retinal hemorrhage, Hearing loss, Hypertension, Wheezing, Gastrointestinal bleeding, Hepatic dysfunction
Rare:
Endocarditis, Otitis externa, Pancytopenia, Anaphylaxis, Systemic lupus erythematous, Rheumatoid arthritis, Diabetic ketoacidosis, Suicide, Psychotic disorder, Coma, Convulsions, Facial palsy, Optic neuropathy, Papilledema, Retinal vascular disorder, Retinopathy, Corneal ulcer, Myocardial infarction, Congestive heart failure, Cardiomyopathy, Angina, Arrhythmia, Atrial fibrillation, Pericarditis, Supraventricular tachycardia, Cerebral hemorrhage, Vasculitis, Interstitial pneumonitis including fatal outcome, Pulmonary embolism, Peptic ulcer, Pancreatitis, Hepatic failure, Cholangitis, Fatty liver, Myositis, Renal insufficiency, Substance overdose, Aplastic anemia, Idiopathic or thrombotic thrombocytopenic purpura, Vision loss, Stevens-Johnson syndrome, Toxic epidermal necrolysis, Angioedema, Erythema multiforme
Frequency not known:
Sepsis, Pure red cell aplasia, Liver and renal graft rejection, Vogt-Koyanagi-Harada disease, Mania, bipolar disorders, Homicidal ideation, Cerebral ischemia, Serous retinal detachment, Peripheral ischemia, Ischemic colitis, tongue pigmentation, Rhabdomyolysis
Theophylline
A 25% increase in the AUC of theophylline (marker of cytochrome P450 1A2 activity) was observed, demonstrating that peginterferon is an inhibitor of cytochrome P450 1A2 activity. Serum concentrations of theophylline should be monitored and appropriate dose adjustments of theophylline made for patients taking theophylline and Pegaferon® concomitantly. The interaction between theophylline and Pegaferon® is likely to be maximal after more than 4 weeks of Pegaferon® therapy.
Methadone
Treatment with peginterferon 180 micrograms once weekly for 4 weeks was associated with mean methadone levels that were 10% to 15% higher than at baseline. The clinical significance of this finding is unknown; Nonetheless, patients should be monitored for the signs and symptoms of methadone toxicity. Especially in patients on a high dose of methadone, the risk for QTc prolongation should be considered.
Clozapine
Myelosuppressive Agents may enhance the adverse/toxic effect of Clozapine. Specifically, the risk for agranulocytosis may be increased.
NRTIs (e.g. didanosine, stavudine, and zidovudine)
Coadministartion may increase toxicities, such as hematologic toxicities. Cases of hepatic decomposition (some fatal) were observed. Closely monitor for treatment-associated toxicities. Consider dose reduction or discontinuation of therapy if worsening of toxicity occurs. Coadministration of didanosine and peginterferon alfa-2a with ribavirin is contraindicated.
Telbivudine
Peginterferon Alfa-2a may enhance the adverse/toxic effect of Telbivudine. Specifically, the risk for peripheral neuropathy may be increased.
Pegloticase
May diminish the therapeutic effect of Peginterferon Alfa-2a.
Children: Hematologic and biochemical assessments were made at weeks 1, 3, 5, and 8, and then every 4 weeks thereafter; TSH measured every 12 weeks.
Adults: CBC (including hemoglobin, WBC, and platelets) and chemistries (including liver function tests and uric acid) measured at weeks 1, 2, 4, 6, and 8, and then every 4-6 weeks (more frequently if abnormal); TSH measured every 12 weeks.
In addition, the following baseline values were used as entrance criteria in adults:
Platelet count ≥ 90,000/mm 3 (as low as 75,000/mm 3 in patients with cirrhosis or 70,000/mm 3 in patients with CHC coinfected with HIV)
ANC ≥1500/mm 3
Serum creatinine <1.5 times ULN
TSH and T 4 within normal limits or adequately controlled
CD4 + cell count ≥200 cells/mm 3 or CD4 + cell count ≥ 100 cells/mm 3 , but < 200 cells/mm 3 and HIV-1 RNA < 5000 copies/mL in CHC patients coinfected with HIV
Hemoglobin ≥ 12 g/dL for women and ≥ 13 g/dL for men in CHC monoinfected patients
Hemoglobin ≥ 11 g/dL for women and ≥ 12 g/dL for men in CHC patients coinfected with HIV
Serum HCV RNA levels (pretreatment, 12 – and 24 weeks after therapy initiation, 24 weeks after completion of therapy). Note: Discontinuation of therapy may be considered after 12 weeks in patients with HCV (genotype 1) who fail to achieve an early virologic response (EVR) (defined as ≥ 2-log decrease in HCV RNA compared to pretreatment) or after 24 weeks with detectable HCV RNA. Treat patients with HCV (genotypes 2, 3) for 24 weeks (if tolerated) and then evaluate HCV RNA levels.
Prior to treatment, pregnancy screening should occur for women of childbearing age who are receiving treatment or who have male partners who are receiving treatment. In combination therapy with ribavirin, pregnancy tests should continue monthly up to 6 months after discontinuation of therapy. Evaluate for depression and other psychiatric symptoms before and during therapy; baseline eye examination and periodically in patients with baseline disorders; baseline echocardiogram in patients with cardiac disease.
Pegaferon® possesses the in vitro antiviral and anti-proliferative activities that are characteristic of interferon alfa2a. Interferon alfa-2a has antiproliferative and immunomodulatory activities. Exactly how interferon alfa-2a works is not known.
Absorption/Distribution
Time to peak serum (Cmax): Occurs between 72 to 96 hours postdose.
Vd: 6 to 14 liters
Metabolism/Excretion
Rate of elimination: 94 mL/h Half-life elimination: Terminal: 50-160 hours; increased with renal dysfunction
Before use, leave the Pegaferon® syringe to stand until it reaches room temperature. This usually takes between 15 and 30 minutes. Do not remove the syringe’s needle cover while allowing it to reach room temperature.
Only take one dose of Pegaferon® from each syringe.
Pegaferon® is given alone and not mixed with other liquids for injection.
Do not shake Pegaferon® Prolonged vigorous shaking may damage the product. If the product has been shaken vigorously, don’t use it.
Check the syringe, to make sure it is the right dose, has not passed its expiry date, is not damaged, and the liquid is clear and not frozen.
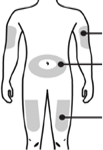
Choose an injection site. Do not inject Pegaferon® into an area that is tender, red, bruised, hard, or has scars or stretch marks. Recommended sites for injection are:
abdomen
thigh
Wash your hands. Use an antiseptic swab on the injection site, to disinfect it.
Hold the pre-filled syringe by the body of the syringe with the covered needle pointing upward.
Do not hold by the plunger head, plunger or needle cover.
Do not pull back on the plunger at any time.
Do not remove the needle cover from the pre-filled syringe until you are ready to inject your Pegaferon®.
Take the cover off the syringe by holding the barrel and pulling the cover off carefully without twisting it. Don’t push the plunger, touch the needle or shake the syringe.
Pinch a fold of skin between your thumb and index finger. Don’t squeeze it.
Push the needle in fully. Your doctor or nurse may have shown you how to do this.
Push the plunger with your thumb as far as it will go to inject the entire amount of liquid. Push it slowly and evenly, keeping the skin fold pinched.
When the plunger is pushed as far as it will go, take out the needle and let go of the skin.
When the needle is pulled out of your skin, there may be a little bleeding at the injection site. This is normal. You can press an antiseptic swab over the injection site for a few seconds after the injection.
Dispose of your used syringe in a safe container.
Pegaferon® possesses the in vitro antiviral and anti-proliferative activities that are characteristic of interferon alfa2a. Interferon alfa-2a has antiproliferative and immunomodulatory activities. Exactly how interferon alfa-2a works is not known.
Store in a refrigerator (2°C – 8°C).
Keep the container in the outer carton in order to protect from light.
Keep out of the reach and sight of children.
Do not use Pegaferon® after the expiry date which is stated on the box and on the syringe label (EXP). The expiry date refers to the last day of that month.
Do not freeze Pegaferon® and do not use Pegaferon® that has been frozen or improperly refrigerated.
Do not shake Pegaferon®.
Pegaferon® pre-filled syringes are single dose containers, discard any unused product.
Sodium chloride, Polysorbate 80, Benzyl alcohol, Sodium acetate, Acetic acid, Water for injections