
About Medicine
Erythropoietin (EPO) is a glycoprotein hormone produced primarily by the kidney in response to hypoxia and is the key regulator of red blood cell (RBC) production. PDpoetin® is a Recombinant human EPO (epoetin alfa), expressed in Chinese hamster ovary cells, and has a 165 amino acid sequence identical to that of human urinary EPO; the 2 are indistinguishable on the basis of functional assays. The apparent molecular weight of erythropoietin is 34000 Dalton.
Pharmaceutical form
Prefilled Syringes: 2000, 4000, 10000 IU in 0.5 mL
Qualitative Composition
Sterile solution, clear and colorless in prefilled syringe.
Guidance to patients
Overdosage
The maximum amount of PDpoetin® that can be safely administered in single or multiple doses has not been determined with respect to the direct effect of epoetin alfa (rch) as distinct from its effect on red cell mass.
The response to PDpoetin® is dose related and individual. With excessive erythropoietic response to PDpoetin®, dosing should be stopped and treatment begun as described above under PRECAUTIONS: Hypertension and Seizures. Phlebotomy may be performed if excessively high hemoglobin levels occur. Additional supportive care should be provided as necessary.
What if you forget to use PDpoetin®?
If you miss a dose of PDpoetin® administer the missed dose as soon as you remember and talk to your doctor about when to use the next doses.
Effect on Ability to Drive and Operate Machinery
No studies on the effects of epoetin alfa on the ability to drive and use machines have been performed.
Use
1. Treatment of symptomatic anemia associated with chronic renal failure (CRF):
– In adults and pediatrics aged 1 to 18 years on hemodialysis and adult patients on peritoneal dialysis.
– In adults with renal insufficiency not yet undergoing dialysis for the treatment of severe anemia of renal origin accompanied by clinical symptoms in patients.
2. Treatment of anemia associated with HIV (zidovudine) therapy when endogenous erythropoietin levels ≤500 mUnits/mL Treatment of anemia due to concurrent myelosuppressive chemotherapy in patients with cancer (nonmyeloid malignancies) receiving chemotherapy (palliative intent) for a planned minimum of 2 additional months of chemotherapy.
3. Indicated in adults in a predonation program to increase the yield of autologous blood. Treatment should only be given to patients with moderate anemia (hemoglobin concentration range between 10 to 13 g/dl, no iron deficiency) if blood saving procedures are not available or insufficient when the scheduled major elective surgery requires a large volume of blood (4 or more units of blood for females or 5 or more units for males).
4. Indicated for non-iron deficient adults prior to major elective orthopedic surgery having a high perceived risk for transfusion complications to reduce exposure to allogeneic blood transfusions. Use should be restricted to patients with moderate anemia (e.g. hemoglobin concentration range between 10 to 13 g/dl) who do not have an autologous predonation program available and with expected moderate blood loss (900 to 1,800 ml).
5. Anemia of prematurity
Dosing: Adult
The recommended desired hemoglobin concentration range is between 10 g/dl to 12 g/dl. PDpoetin® should be administered in order to increase hemoglobin to not greater than 12 g/dl. A rise in hemoglobin of greater than 2 g/dl over a four week period should be avoided. If it occurs, appropriate dose adjustment should be made as provided.
Due to intra-patient variability, occasional individual hemoglobin values for a patient above and below the desired hemoglobin concentration range may be observed. Hemoglobin variability should be addressed through dose management, with consideration for the hemoglobin concentration range of 10 g/dl to 12 g/dl.
A sustained hemoglobin level of greater than 12 g/dl should be avoided. If the hemoglobin is rising by more than 2 g/dl per month, or if the sustained hemoglobin exceeds 12 g/dl reduce the PDpoetin® dose by 25%. If the hemoglobin exceeds 13 g/dl, discontinue therapy until it falls below 12 g/dl and then reinstitute PDpoetin® therapy at a dose 25% below the previous dose.
Patients should be monitored closely to ensure that the lowest approved dose of PDpoetin® is used to provide adequate control of anemia and of the symptoms of anemia.
Treatment with PDpoetin® is divided into two stages – correction and maintenance phase.
1. Adult hemodialysis patients
Correction phase:
The starting dose is 50 IU/kg, 3 times per week.
If necessary, increase or decrease the dose by 25 IU/kg (3 times per week) until the desired hemoglobin concentration range between 10 g/dl to 12 g/dl is achieved (this should be done in steps of at least four weeks).
Maintenance phase:
The recommended total weekly dose is between 75 IU/kg and 300 IU/kg.
Appropriate adjustment of the dose should be made in order to maintain hemoglobin values within the desired concentration range between 10 g/dl to 12 g/dl.
Patients with very low initial hemoglobin (< 6 g/dl) may require higher maintenance doses than patients whose initial anemia is less severe (> 8 g/dl).
2. Adult patients with renal insufficiency not yet undergoing dialysis
Correction phase:
Starting dose of 50 IU/kg, 3 times per week, followed if necessary by a dosage increase with 25 IU/kg increments (3 times per week) until the desired goal is achieved (this should be done in steps of at least four weeks).
Maintenance phase:
During the maintenance phase, PDpoetin® can be administered either 3 times per week, and in the case of subcutaneous administration, once weekly or once every 2 weeks.
Appropriate adjustment of dose and dose intervals should be made in order to maintain hemoglobin values at the desired level: hemoglobin between 10 g/dl and 12 g/dl). Extending dose intervals may require an increase in dose.
The maximum dosage should not exceed 150 IU/kg 3 times per week, 240 IU/kg (up to a maximum of 20,000 IU) once weekly, or 480 IU/kg (up to a maximum of 40,000 IU) once every 2 weeks.
3. Adult peritoneal dialysis patients
Correction phase:
The starting dose is 50 IU/kg, 2 times per week.
Maintenance phase:
The recommended maintenance dose is between 25 IU/kg and 50 IU/kg, 2 times per week in 2 equal injections.
4. Treatment of adult surgery patients in an autologous predonation program
Mildly anemic patients (hematocrit of 33 to 39%) requiring predeposit of ≥ 4 units of blood should be treated with PDpoetin® 600 IU/kg intravenously, 2 times per week for 3 weeks prior to surgery. PDpoetin® should be administered after the completion of the blood donation procedure.
5. Treatment of adult patients scheduled for major elective orthopedic surgery
The recommended dose is PDpoetin® 600 IU/kg administered subcutaneously weekly for three weeks (days 21, 14 and 7) prior to surgery and on the day of surgery.
In cases where there is a medical need to shorten the lead time before surgery to less than three weeks, PDpoetin® 300 IU/kg should be administered subcutaneously daily for 10 consecutive days prior to surgery, on the day of surgery and for four days immediately thereafter.
If the hemoglobin level reaches 15 g/dl, or higher, during the perioperative period, administration of PDpoetin® should be stopped and further dosages should not be administered.
Dose adjustment to maintain hemoglobin concentrations between 10 g/dl to 12 g/dl
If the hemoglobin concentration is increasing by more than 2 g/dl per month, or if the hemoglobin concentration level exceeds 12 g/dl, reduce the PDpoetin® dose by about 25 to 50%.
If the hemoglobin concentration level exceeds 13 g/dl, discontinue therapy until it falls below 12 g/dl and then reinitiate PDpoetin® therapy at a dose 25% below the previous dose.
Patients should be monitored closely to ensure that the lowest approved dose of erythropoiesis-stimulating agent (ESA) is used to provide adequate control of the symptoms of anemia.
PDpoetin® therapy should continue until one month after the end of chemotherapy.
Dosing: Pediatric
1. Anemia of prematurity
I.V., SubQ: Dosing range: 500-1250 units/kg/ week divided into 2-5 doses for 10 doses; commonly used dose: 250 units/kg/dose 3 times weekly for 10 doses; supplement with oral iron therapy 3-8 mg/kg/day.
2. Treatment of symptomatic anemia in chronic renal failure patients on hemodialysis
In pediatric patients the recommended hemoglobin concentration range is between 9.5 g/dl to 11 g/dl. PDpoetin® should be administered in order to increase hemoglobin to not greater than 11 g/dl. A rise in hemoglobin of greater than 2 g/dl over a four week period should be avoided. If it occurs, appropriate dose adjustment should be made as provided.
Patients should be monitored closely to ensure that the lowest approved dose of PDpoetin® is used to provide adequate control of anemia and of the symptoms of anemia.
Treatment with PDpoetin® is divided into two stages – correction and maintenance phase.
In pediatric patients on hemodialysis where intravenous access is readily available, administration by the intravenous route is preferable.
Correction phase:
The starting dose is 50 IU/kg intravenously, 3 times per week.
If necessary, increase or decrease the dose by 25 IU/kg (3 times per week) until the desired hemoglobin concentration range of between 9.5 g/dl to 11 g/dl is achieved (this should be done in steps of at least four weeks).
Maintenance phase:
Appropriate adjustment of the dose should be made in order to maintain hemoglobin levels within the desired concentration range between 9.5 g/dl to 11 g/dl.
Generally, children less than 30 kg require higher maintenance doses than children over 30 kg and adults.
Pediatric patients with very low initial hemoglobin may require higher maintenance doses than patients whose initial hemoglobin is higher (> 6.8 g/dl).
3. Treatment of pediatric patients with chemotherapy-induced anemia
The safety and efficacy of PDpoetin® in pediatric patients receiving chemotherapy have not been established.
4. Treatment of pediatric surgery patients in an autologous predonation program
The safety and efficacy of PDpoetin® in pediatrics have not been established. No data are available.
5. Treatment of pediatric patients scheduled for major elective orthopedic surgery
The safety and efficacy of PDpoetin® in pediatrics have not been established. No data are available.
Contraindications
- Uncontrolled hypertension
- Known sensitivity to human albumin
- Known sensitivity to mammalian cell derived products
- Hypersensitivity to the active substance or to any of the excipients
- Patients who develop Pure Red Cell Aplasia (PRCA) following treatment with any erythropoietin should not receive PDpoetin® or any other erythropoietin.
- Surgery patients who for any reason cannot receive adequate antithrombotic prophylaxis or treatment.
- Patients scheduled for elective surgery, who are not participating in an autologous blood pre-deposit program and who have severe coronary, peripheral arterial, carotid or cerebral vascular disease, including patients with recent myocardial infarction or cerebral vascular accident.
- Epoetin alfa is not approved in the management of anemia associated with hepatitis C.
- History of Thromboembolism
- If you have cancer and you will not be receiving chemotherapy that may cause anemia.
- If you have a cancer that has a high chance of being cured.
- In place of emergency treatment for anemia (RBC transfusions).
Warning and Precautions
1. In all patients receiving epoetin alfa, blood pressure should be closely monitored and controlled as necessary. Epoetin alfa should be used with caution in the presence of untreated, inadequately treated or poorly controllable hypertension. It may be necessary to add or increase anti-hypertensive treatment. If blood pressure cannot be controlled, epoetin alfa treatment should be discontinued.
2. Hypertensive crisis with encephalopathy and seizures, requiring the immediate attention of a physician and intensive medical care, have occurred also during epoetin alfa treatment in patients with previously normal or low blood pressure. Particular attention should be paid to sudden stabbing migraine-like headaches as a possible warning signal.
Epoetin alfa should be used with caution in patients with epilepsy, history of seizures, or medical conditions associated with a predisposition to seizure activity such as CNS infections and brain metastases.3. Epoetin alfa should be used with caution in patients with chronic liver failure. The safety of epoetin alfa has not been established in patients with hepatic dysfunction.
4. An increased incidence of thrombotic vascular events (TVEs) has been observed in patients receiving ESAs.These include venous and arterial thrombosis and embolism (including some with fatal outcomes), such as deep venous thrombosis, pulmonary embolism, retinal thrombosis, and myocardial infarction.
5. Epoetins are growth factors that primarily stimulate red blood cell production. Erythropoietin receptors may be expressed on the surface of a variety of tumor cells. As with all growth factors, there is a concern that epoetins could stimulate the growth of tumors. In several controlled studies, epoetins have not been shown to improve overall survival or decrease the risk of tumor progression in patients with anemia associated with cancer.
Pregnancy
There are no adequate and well-controlled studies in pregnant women. Studies in animals have shown reproduction toxicity. Consequently, epoetin alfa should be used in pregnancy only if the potential benefit outweighs the potential risk to the fetus. The use of epoetin alfa is not recommended in pregnant surgical patients participating in an autologous blood predonation.
Lactation
It is not known whether exogenous epoetin alfa is excreted in human milk. Epoetin alfa should be used with caution in nursing women. A decision on whether to continue/discontinue breast-feeding or to continue/discontinue therapy with epoetin alfa should be made taking into account the benefit of breast-feeding to the child and the benefit of epoetin alfa therapy to the woman.
The use of epoetin alfa is not recommended in lactating surgical patients participating in an autologous blood predonation program.
Children
Incomplete information is available, particularly on the rate of change of hemoglobin and blood pressure in neonate under 1 month.
Adverse Effects
PDpoetin® generally causes few side effects and helps most people with anemia; however it may have some unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
System Organ Class Frequency Very common Common Uncommon Rare Very rare Not Known Blood and lymphatic system disorders Erythropoietin antibody-mediated pure red cell aplasia, Thrombocythemia Metabolism and nutrition disorders Hyperkalemia Immune system disorders Anaphylactic reaction, Hypersensitivity Nervous system disorders Headache Convulsions Vascular disorders Venous and arterial thrombosis Hypertension Hypertensive crisis Respiratory, thoracic and mediastinal disorders Cough Respiratory tract congestion Gastrointestinal disorders Diarrhea, Nausea, Vomiting Skin and subcutaneous tissue disorders Rash Angioneurotic edema,Urticaria Musculoskeletal and connective tissue disorders Arthralgia, Bone pain, Myalgia, Pain in extremity Congenital and familial/genetic disorders Porphyria General disorders and administration site conditions Pyrexia Chills, Influenza-like illness, Injection site reaction, Edema peripheral Drug ineffective Frequency estimate: Very common (≥ 1/10), Common (≥ 1/100 to < 1/10), Uncommon (≥ 1/1,000 to < 1/100), Rare (≥ 1/10,000 to < 1/1,000), Very Rare (< 1/10,000), Not known
Drug Interactions
Thalidomide ↔ Epoetin alfa
Using thalidomide together with epoetin alfa may increase the risk of dangerous blood clots. The risk is higher if you are using thalidomide with dexamethasone or other chemotherapy drugs for the treatment of multiple myeloma than if you are using thalidomide alone for some other condition. Also, the risk is increased with age, cigarette smoking, high blood pressure, or high cholesterol. You should seek immediate medical attention if you experience potential signs and symptoms of blood clots such as chest pain, shortness of breath, difficulty breathing, coughing up blood, sudden loss of vision, and/or pain, redness or swelling in an arm or leg. It is important to tell your doctor about all other medications you use, including vitamins and herbs. Do not stop using any medications without first talking to your doctor.
Cyclosporine ↔ Epoetin alfa
Both cyclosporine and epoetin alfa can increase blood pressure, and the effect may be greater when these medications are combined. In addition, because epoetin alfa affects red blood cells, it may alter the blood levels of cyclosporine. You may need to have your blood pressure and drug levels checked more frequently by your doctor to safely use both medications. Contact your doctor if your condition changes or you experience increased side effects of cyclosporine. It is important to tell your doctor about all other medications you use, including vitamins and herbs. Do not stop using any medications without first talking to your doctor.
Angiotensin-converting enzyme (ACE) inhibitors ↔ Epoetin alfa
Angiotensin-converting enzyme (ACE) inhibitors may interfere with the effects of both endogenous and exogenous erythropoietin.
Heparin↔ Epoetin alfa
Adjustments in the dialysis prescription and increased anticoagulation with heparin may be required to prevent clotting of the extracorporeal circuit during hemodialysis.
Monitoring Parameters
Evaluate transferrin saturation and serum ferritin prior to and during PDpoetin® Iron therapy when serum ferritin is less than 100 mcg/L or when serum transferrin saturation is less than 20%.
The majority of patients with CKD will require supplemental iron during the course of ESA therapy. Following initiation of therapy and after each dose adjustment, monitor hemoglobin weekly until the hemoglobin level is stable and sufficient to minimize the need for RBC transfusion.
It is recommended that the platelet count is regularly monitored during the first 8 weeks of therapy.
In chronic renal failure patients the rate of increase in hemoglobin should be approximately 1 g/dl per month and should not exceed 2 g/dl per month to minimize risks of an increase in hypertension.
In patients developing sudden lack of efficacy defined by a decrease in hemoglobin (1 to 2 g/dl per month) with increased need for transfusions, a reticulocyte count should be obtained and typical causes of non-response (e.g. iron, folate or Vitamin B12deficiency, aluminium intoxication, infection or inflammation, blood loss, hemolysis and bone marrow fibrosis of any origin) should be investigated.
Mechanism of action
Erythropoietin (EPO) is a glycoprotein hormone produced primarily by the kidney in response to hypoxia and is the key regulator of red blood cell (RBC) production. EPO is involved in all phases of erythroid development, and has its principal effect at the level of erythroid precursors. After EPO binds to its cell surface receptor, it activates signal transduction pathways that interfere with apoptosis and stimulates erythroid cell proliferation.
Pharmacokinetics
Absorption: About 20% in SC route. After subcutaneous administration, Cmax was achieved within 5 to 24 hours.
Distribution: About 9 L
Elimination: In adult and pediatric patients with CKD, the elimination half-life (t1/2) of plasma erythropoietin after intravenous administration of PDpoetin® ranged from 4 to 13 hours.
In Anemic cancer patients the elimination half-life (t1/2) of plasma erythropoietin after intravenous administration of PDpoetin® ranged from 16-67 hours.
The pharmacokinetic profile of PDpoetin® in children and adolescents appeared similar to that of adults.
The pharmacokinetics of PDpoetin® has not been studied in patients with HIV infection.
Guidance for Injection
PDpoetin® can be injected into your body using two different ways (routes) as described below. Follow your healthcare provider’s instructions about how you should inject PDpoetin®. In patients on hemodialysis, the intravenous (IV) route is recommended.
Before use, leave the PDpoetin® syringe to stand until it reaches room temperature. This usually takes between 15 and 30 minutes. Do not remove the syringe’s needle cover while allowing it to reach room temperature.
Only take one dose of PDpoetin® from each syringe.
PDpoetin® is given alone and not mixed with other liquids for injection.
Do not shake PDpoetin® Prolonged vigorous shaking may damage the product. If the product has been shaken vigorously, don’t use it.
Check the syringe, to make sure it is the right dose, has not passed its expiry date, is not damaged, and the liquid is clear and not frozen.
Choose an injection site. Do not inject PDpoetin® into an area that is tender, red, bruised, hard, or has scars or stretch marks. Recommended sites for injection are:
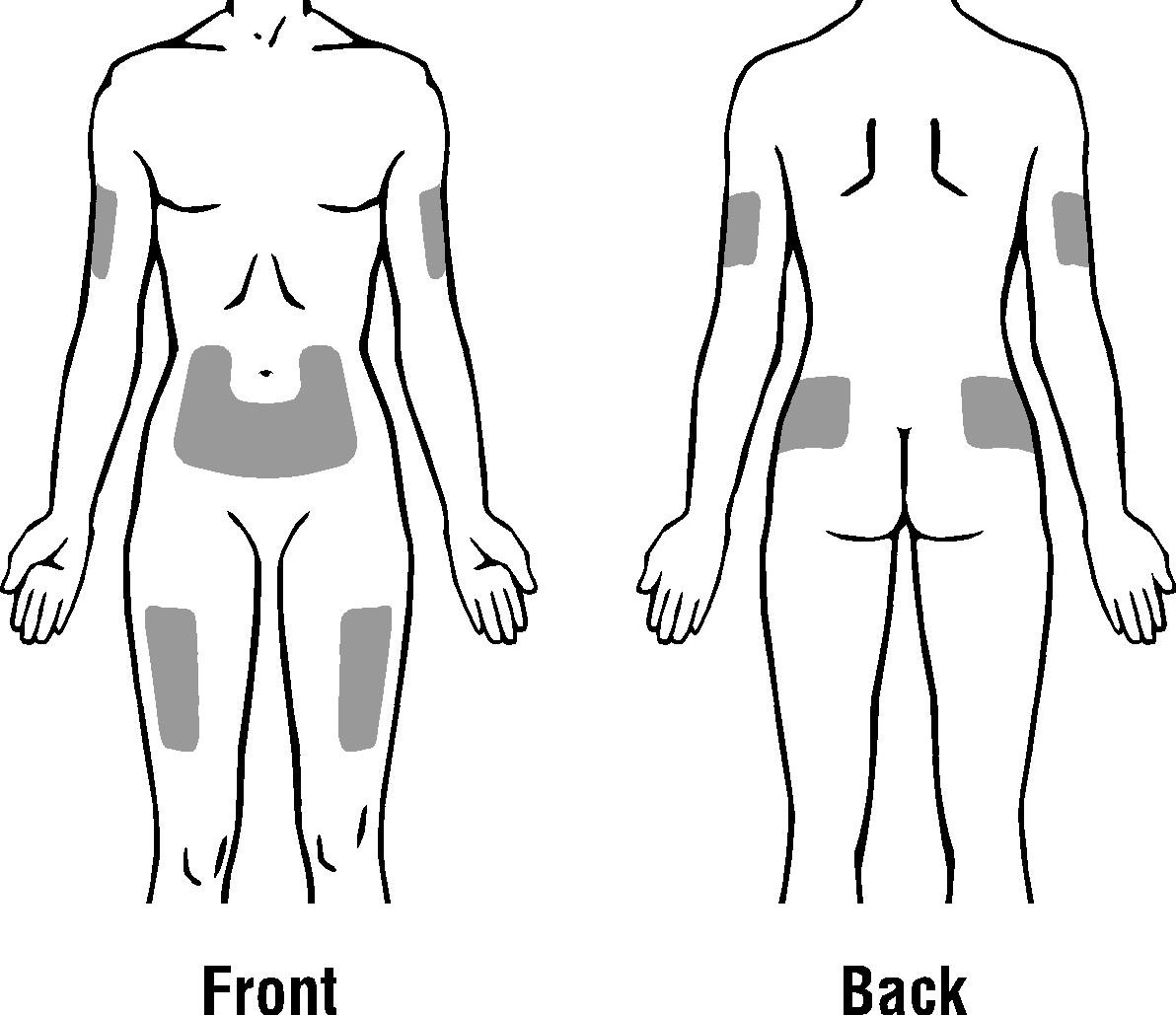
The outer area of the upper arms
The abdomen (except for the 2-inch area around the navel)
The front of the middle thighs
The upper outer area of the buttocks
Subcutaneous Route:
- Wash your hands. Use an antiseptic swab on the injection site, to disinfect it.
- Hold the pre-filled syringe by the body of the syringe with the covered needle pointing upward.
- Do not hold by the plunger head, plunger or needle cover.
- Do not pull back on the plunger at any time.
- Do not remove the needle cover from the pre-filled syringe until you are ready to inject your PDpoetin®.
- Take the cover off the syringe by holding the barrel and pulling the cover off carefully without twisting it. Don’t push the plunger, touch the needle or shake the syringe.
- Pinch a fold of skin between your thumb and index finger. Don’t squeeze it.
- Push the needle in fully. Your doctor or nurse may have shown you how to do this.
- Push the plunger with your thumb as far as it will go to inject the entire amount of liquid. Push it slowly and evenly, keeping the skin fold pinched.
- When the plunger is pushed as far as it will go, take out the needle and let go of the skin.
- When the needle is pulled out of your skin, there may be a little bleeding at the injection site. This is normal. You can press an antiseptic swab over the injection site for a few seconds after the injection.
- Dispose of your used syringe in a safe container.
Intravenous Route:
PDpoetin® can be injected in your vein through a special access port placed by your healthcare provider. This type of PDpoetin® injection is called an intravenous (IV) injection. This route is usually for hemodialysis patients.
- Wipe off the venous port of the hemodialysis tubing with an alcohol wipe.
- Insert the needle of the syringe into the cleaned venous port and pushes the plunger all the way down to inject all the PDpoetin®.
- Remove the syringe from the venous port. Do not recap the needle.
- Dispose of the used syringe and needle.
- Administer over at least one to five minutes, depending on the total dose.
- In hemodialysed patients, a bolus injection may be given during the dialysis session through a suitable venous port in the dialysis line. Alternatively, the injection can be given at the end of the dialysis session via the fistula needle tubing, followed by 10 ml of isotonic saline to rinse the tubing and ensure satisfactory injection of the product into the circulation.
- A slower administration is preferable in patients who react to the treatment with “flu-like” symptoms.
- Do not administer PDpoetin® by intravenous infusion or in conjunction with other drug solutions.
Storage and Stability
- Store in a refrigerator (2°C – 8°C).
- Keep the container in the outer carton in order to protect from light.
- Keep out of the reach and sight of children.
- Do not use PDpoetin® after the expiry date which is stated on the box and on the syringe label (EXP). The expiry date refers to the last day of that month.
- Do not freeze PDpoetin® and do not use PDpoetin® that has been frozen or improperly refrigerated.
- Do not shake PDpoetin®.
- PDpoetin® pre-filled syringes are single dose containers, discard any unused product.
Other Composition
Human Albumin – Sodium Chloride – Sodium Citrate
